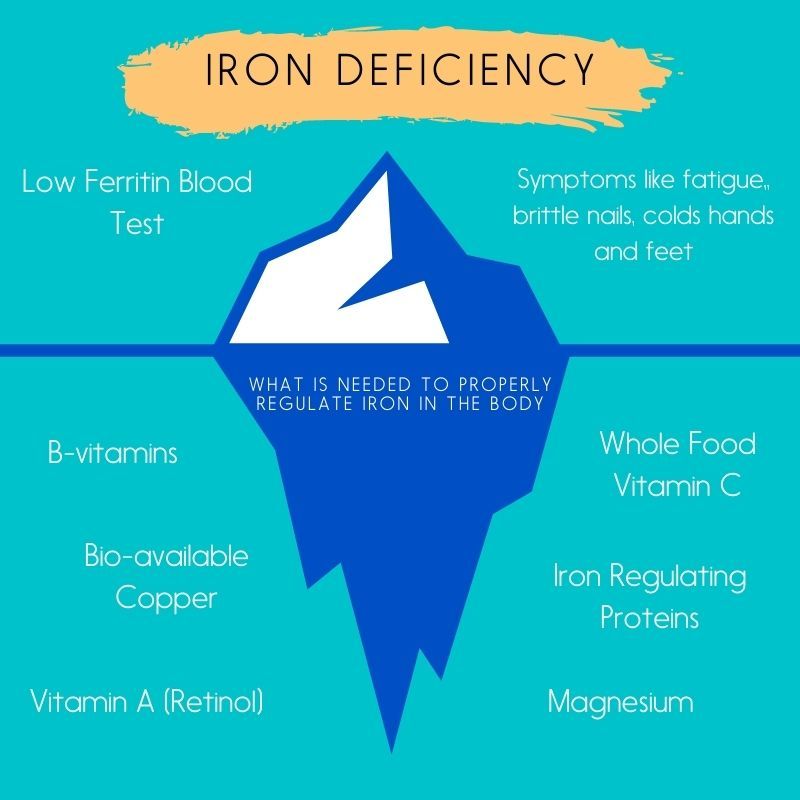
Many of us have been told that we are deficient in iron and the general solution is to increase iron-rich foods and/or to take a supplement. While true Iron deficiency anemia does exist (found with specific blood tests), there is typically a lot more that is going on behind the scenes.
As most things in life, finding the right balance is crucial. Iron, in excess, can be toxic. “Many recent studies show that it is involved in degenerative brain diseases, such as Parkinson's, ALS (Lou Gehrig's disease), Huntington's chorea, and Alzheimer's disease. Iron is now believed to have a role in skin aging, atherosclerosis, and cataracts of the lenses of the eyes, largely through its formation of the age pigment (oxidized mass of unsaturated fat and iron, formed by uncontrolled free radicals.” Dr. Ray Peat.
While iron is an essential nutrient for humans, it also feeds pathogenic microbes (1). If you have an excess of iron stored in your cells/tissues (more explanation about this below), there is a greater chance of proliferation among unfriendly bacteria, yeast, and viruses. For example, one study showed that infants given an iron-fortified cow’s milk preparation had lower Bifidobacterium but higher counts of Bacteroides and E. coli than infants receiving an unfortified cow’s milk preparation (2).
Let’s look at iron deficiency from another angle to try and understand why this occurs and some solutions that help support iron metabolism.
The Importance of Copper for Iron Regulation
Iron is predominately regulated by bio-available copper and Vitamin A (more on this below). The Iron recycling system (RES) is comprised mainly of macrophages and monocytes which play a role in iron metabolism. Without the presence of copper keeping the iron recycling system in check, iron can become stored in our tissues causing oxidative stress throughout the body. Don’t get me wrong, iron is important! 70% of our bodies iron is found in red blood cells, know as hemoglobin. Hemoglobin is essential for transferring oxygen in our blood to the lungs and tissues. But copper actually gives us the ability to access the oxygen we need to feed the cells in these systems.
But Isnt Copper Toxic?
Excess unbound Copper (Cu) on it’s own can be toxic. “This unusable form must go through a conversion to be bio-available and usable in the body. Ceruloplasmin is the transport protein produced in the liver that transports Copper throughout the body and makes copper bio-available. For the copper to be bound to ceruloplasmin, there must be Vitamin A in the form of retinol, which is only found in animal fats. Retinol A is required to load copper into ceruloplasmin, otherwise the copper will not be beneficial to the body. Ceruloplasmin converts the toxic form of iron into it’s transportable, beneficial form transferrin (Fe +3), the iron transport protein, then transports the iron out of the tissues. When there isn’t enough activated ceruloplasmin, the iron gets stored in the tissues and cells, mainly in the mitochondria. The iron then reacts with oxygen causing oxidative stress and inflammation. ” lajesky inspired by Morley Robbins, MBA, CHC.
To sum it up, too little copper can make us store excess iron in the tissues and too much iron can block our absorption of copper.


Vitamin C and Iron Regulation
Whole food Vitamin C contains an enzyme called tyrosinase which stimulates the liver to produce bio-available copper (ceruloplasmin).
A note on Ascorbic Acid: It does not contain the same cofactors as whole food vitamin C and can deplete the adrenals glands of Vitamin C stores. Most ascorbic acid is derived from corn and is commonly synthesized using volatile acids. Not only has high doses of Ascorbic acid been linked to the formation of kidney stones, but one animal study caused reduced concentrations of copper and ceruloplasmin in the liver from high amounts of AA (3).
What if my blood test is low in iron?
Typical blood tests usually look at Ferritin levels (a blood protein that contains iron). While ferritin should be on the lower end, anything below 21 might indicate a bio-available copper deficiency. And to make this more complicated, if inflammation is or has been present, more iron is being pushed and stored in the tissues. This causes oxidative stress at the cellular level, which leads to further inflammation. Therefore, further investigation is important and why “just taking an iron supplement” might not be the best course of action. A thorough test to consider is the Full Monte Iron Panel. Under a qualified practitioners guidance, this test can help you see the full picture and get to the root cause of your iron deficiency.
What Causes Copper and Iron Dysregulation?
A few reasons include:
• Glyphosate and other herbicides & pesticides
• Too much synthetic Vitamin D, A, Zinc, and Calcium Supplements (whole food supplements are ideal)
• Excess phytates (nuts/seeds, beans, legumes, etc)
• Iron fortified foods
• Drinking and Bathing in unfiltered water
• Pharmaceuticals (i.e. birth control)
• High fructose corn syrup
Ways to Properly Regulate Iron in the Body
• Consume foods high in copper and Vitamin A to support your RES (liver, bee pollen, shellfish, mushrooms, potatoes, grass-fed dairy, fruits, and fatty fish)
• Increase intake of polyphenols
• Include non-synthetic supplements like Vitamin E*, whole food Vitamin C*, liver, and Fiber
• Regulating your menstrual cycle
• Support thyroid and liver function (it’s all connected)
• STRESS REDUCTION
*Take away from foods that contain iron
So if you are considering an iron supplement, it’s important to make sure you are getting the proper nutrients to support iron metabolism and regulation. Personally, I think whole food and liver options are ideal, but always speak to your practitioner and get detailed bloodwork done before starting an iron supplement.
All information and tools presented and written within this Article are for educational and Informational purposes only. Any nutrition, lifestyle and product recommendations are not intended to diagnose, treat, cure, or prevent any disease. Before starting any new supplements, diet and exercise program please check with your doctor or practitioner.
Sources:
https://therootcauseprotocol.com/resources/
http://raypeat.com/articles/articles/iron-dangers.shtml
(1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3676888/
(2) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6315993/
(3) https://pubmed.ncbi.nlm.nih.gov/1493135/


